
Ø 2016年會議背景
在過去的10年中,全球仿制藥市場發展的增速是專利藥的2倍以上。今后幾年,將是藥品專利到期的高峰,2014-2018年預計將有1295個仿制藥到期,影響197億美元銷售額。
——數據來自《2015-2020年*化學原料藥行業產銷需求與投資預測分析報告》
而在*,藥審改革不斷加速,仿制藥政策變化加劇,對仿制藥行業的整體發展產生了重要影響:
關于開展仿制藥質量和療效一致性評價的意見(征求意見稿)出臺;
仿制藥的申報從兩報兩批變為一報一批,仿制藥的獲批速度將大大加快;
仿制藥一致性評價*新進展,CFDA發布三大指導原則;
史上*嚴“藥物臨床試驗數據自查令”如火如荼執行……
根據食藥監總局統計,我國有近5000家藥企,仿制藥企業占90%以上。2018年前后,*的仿制藥市場將會在政策變化和競爭加劇的情況下,發生重大調整。
2016年*仿制藥峰會將集合CPhI全球資源和前五屆峰會的精華,進一步挖掘和探討仿制藥政策法規、市場與競爭、研發與技術方面的重點,為國內外從事仿制藥領域的專業人士提供交流與展示的平臺,促進國際合作與互動。
Ø 會議架構
|
會議第一天(2016年4月12日) |
第一章:政策探討 |
|
第二章:市場與競爭 |
|
會議第二天(2016年4月13日) |
第三章:研發與技術 |
Ø 參會公司及部門
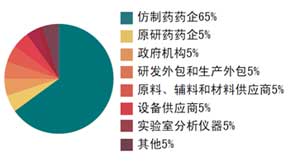
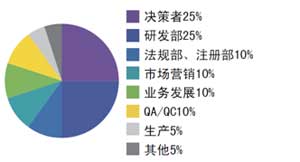
Ø 往屆部分參會企業
梯瓦,輝瑞,諾華,勃林格殷格翰,國藥,上藥,華海藥業,揚子江藥業,石藥集團,上海恒瑞醫藥,先聲藥業,海正藥業,華北制藥,華東醫藥,齊魯制藥,雅培,邁蘭,山德士,阿斯利康,賽諾菲,葛蘭素史克,強生,百時美施貴寶,拜耳,羅氏,衛材,武田,葵花藥業,海思科,四川科倫,昆明制藥,浙江醫藥,武漢人福,東北制藥,海南諾爾康,新疆新姿源,聯化科技,上海騰瑞制藥,和記黃埔,羅欣藥業,南京藥石,浙江仙琚,上海騰瑞,上海特化,江蘇恩華藥業,海正輝瑞,上海欣昌,朗圣藥業,中化藍天,羅氏制藥,上海海尼藥業,上海現代制藥,中信國健,大連美羅藥業,廣東眾生藥業,藥明康德,正大天晴,麗珠制藥,亞寶藥業,哈藥集團,深圳華力康……
>>請參見完整往屆參會名單,含參會人員職位名稱
Ø 2016會議議程
|
會議第一天4月12日 |
|
0830-0900 |
注冊簽到 |
|
第一章:政策探討 |
|
0900-0910 |
開幕致辭 |
|
0910-0950 |
解讀與應對:仿制藥質量和療效一致性評價
一致性評價政策更新的核心內容解讀:參比制劑遴選與研究方法選擇
一致性評價政策更新對于藥品生產企業產品的影響
政策變化中的國內仿制藥市場競爭格局變化 |
|
0950-1030 |
新政實施與展望:仿制藥企業獲得的利好機會
仿制藥生物等效性試驗由審批改為備案制,縮短仿制藥開發周期
仿制藥申報從兩報兩批變為一報一批,獲批速度加快
新政的實施展望,及新政環境下仿制藥市場的競爭態勢分析 |
|
1030-1050 |
茶歇 |
|
1050-1130 |
藥品上市許可人制度(MAH)能否在*之行?
藥品上市許可人制度的核心內容解讀
藥品上市許可人制度對于藥品研發和創新的積極意義
在*實行藥品上市許可人制度的可行性分析 |
|
1130-1200 |
政策變換下的行業整合,CRO企業面臨的機會與挑戰
政策利好,研發外包服務受捧,CRO企業迎來新的發展機會
臨床試驗數據自查核查,CRO企業將承擔更多責任
在新的政策挑戰下,CRO企業如何與藥企合作,互利互惠 |
|
1200-1240 |
小組討論:國內政策的變化以及仿制藥產業鏈內企業的發展出路
美國、歐洲和日本仿制藥審評政策的優勢借鑒
仿制藥臨床應用、招標采購、醫保報銷等配套政策的實施展望
藥品生產企業、CRO企業、輔料等其他產業鏈內企業的發展困惑、瓶頸與未來 |
|
1240-1400 |
午餐 |
|
第二章:市場與競爭 |
|
1400-1440 |
轉型中的國內外仿制藥市場分析
質量一致性評價對于仿制藥市場的影響
國內外仿制藥市場發展現狀及趨勢預估
變化的發展環境與戰略調整:首仿藥與*仿制藥戰略 |
|
1440-1520 |
戰略合作與投資并購,仿制藥企業如何探求新發展
仿制藥市場的資本運作現狀
通過戰略合作與投資并購,實現價值*大化
未來產業的發展趨勢預測 |
|
1520-1540 |
茶歇 |
|
1540-1620 |
*仿制藥企業進入歐美市場:瓶頸與機會
美國市場的仿制藥需求和政策環境機會
*仿制藥企業出口歐美市場面臨的問題分析
如何突破瓶頸,促進制劑出口升級 |
|
1620-1700 |
*仿制藥國際化策略探討
研發和產品質量上的差距與提升
海外市場的營銷與品牌戰略
如何制定國際化策略 |
|
會議第二天 4月13日 |
|
0830-0900 |
注冊簽到 |
|
第三章:研發與技術 |
|
0900-0940 |
仿制藥開發過程中的專利戰略
知識產權保護對于仿制藥企業的重要性意義
仿制藥搶仿開發過程中的知識產權糾紛
仿制藥企業如何應對知識產權糾紛,促進立項和藥品研發 |
|
0940-1020 |
仿制藥研發過程中的創新策略與方法
立項的關鍵點和策略
仿制藥研發中的關鍵技術要點
仿制藥產品的質量控制方法 |
|
1020-1040 |
茶歇 |
|
1040-1120 |
藥用輔料標準明晰,輔料企業的提升與發展空間探討
2015版藥典新增的藥用輔料有標準解讀
關鍵輔料的質量控制對制劑研發的重要性
輔料企業在核心技術、產品質量和服務方面的提升與應對 |
|
1120-1220 |
仿制藥雜質控制策略和具體方法
雜質控制在仿制藥申請中的法規要求
CMC研究中的雜質問題
仿制藥雜質控制的具體策略 |
|
1220-1330 |
午餐 |
|
1330-1410 |
小組討論:仿制藥研發中選擇BE實驗還是體外溶出試驗
國外相關標準的借鑒意義
監管部門的要求和產品申請過程中會遇到的問題
<, SP, style="FONT-FAMILY: '微軟雅黑','sans-serif'; mso-ascii-font-family: Calibri; mso-ascii-theme-font: minor-latin; mso-hansi-font-family: Calibri; mso-hansi-theme-font: minor-latin" AN>兩種方法的利弊分析 |
|
1410-1510
|
仿制藥溶出度曲線的測定與比較
原研藥品的多條溶出度曲線測定方法
制定質量標準中的各個參數
溶出度曲線測定過程中的技術要點 |
|
1510-1530 |
茶歇 |
|
1530-1700 |
仿制藥生物等效性(BE)試驗的指導原則與具體操作
藥物制劑人體生物等效性試驗指導原則解讀
如何準備BE備案資料
BE試驗資源現狀分析及拓展途徑
上市產品再評價申請BE
如何保證BE的試驗的真實性和規范性 |
|
1700-1710 |
會議結束語 |
Ø 主辦方介紹
博聞*旗下的全資子公司及合資公司于*內地多個主要城市設有10個辦事處,包括北京、上海、廣州、杭州、廣州和深圳。公司提供逾60種產品及服務,包括商貿會展、會議、雜志、網站及培訓,產業共涵蓋16個市場領域。博聞*是*大陸市場之*大的商營展會主辦商,舉辦多個*首要的展會,當中大部分更屬亞洲*大型或全球第二大型的展會。公司舉辦53個商貿展會、10個會議、出版6本高質專業雜志,以及營運6個垂直網站,為來自國內及全球數以萬計的參展商、買家、會議代表、廣告商、讀者及合作伙伴提供高質素的會面商貿配對、由業界**主持的會議、即時市場動向及新聞、網上貿易網絡及采購和市場推廣平臺。公司在*共有超過550名員工。
CPhI是UBM在醫藥領域的*展會品牌,母展發源于歐洲。CPhI是**大的制藥原料藥博覽會,每年6月再上海新國際博覽中心全館召開。依托于CPhI的強大號召力,CPhI Conferences致力于通過高標準的專業會議向醫藥行業*客戶傳遞*新法規政策、市場發展動態、典型案例分析、*新研發技術以及更多的溝通合作機會。CPhI Conferences目前已在歐洲、*、印度、南美四大區域成功舉辦多場醫藥行業的*會議。
Ø 酒店地址
上海巴黎春天新*酒店,3樓宴會廳
地址:上海市長寧區定西路1555號
In the past 10 years, the market development growth of generics was twice as much as innovator drugs. In the next few years, there will be a drug patents expire peak. 1295 generic drugs will be off-patent during the year 2014 to 2018, which will affect $19.7 billion in total.
– Data from Analysis report on production and marketing of chemical pharmaceutical industry in China during 2015-2020.
There are lot of policy and regulation updates in China according to CFDA measurement. The new policy will influence the whole generics industry chain in perspective of generics application, quality improvement, clinical data requirement and internationalization.
Based on the data analysis from CFDA that there are around 5,000 pharmaceutical manufacturers in China in total, and 90% of them manufacture generics. There will be big changes and great adjustment around the year of 2018 due to the changeable policy and market environment.
The 6th NEXTGEN CHINA 2016 will gather all resource of CPhI global and essence of past five conferences to explore topics above and showcase evolving generics landscape & solutions.
Ø Conference Structure
|
Day One (April 12, 2016) |
|
|
Chapter Two: Market and Competition |
|
Day Two (April 13, 2016) |
Chapter Three: R&D and Technology |
Ø Who Should Attend
Ø Past Attendees
Teva, Mylan, Sandoz, Pfizer, GSK, Boehringer-Ingelheim, AstraZeneca, Sanofi, Movartis, Merck, JNJ, Abbott, Bristol-Myers Squibb, Bayer, Roche, Dr. Reddy’s, Shanghai Pharma Group, Huhai, Hengrui, Yangtze River, Tasly, China Resources, Xian-Janssen, Qilu, Zhejiang Medicine, Wuhan Humanwell, Northeast Pharm, North China Pharmaceutical, Sinopharm, Kelun Group…
>>Please refer to the whole list of past attendee including Name, Job title Company
Ø 2016 Agenda
|
Conference Day One |
|
0830-0900 |
Registration & Networking |
|
Chapter One: Regulation and Policy Discussion |
|
0900-0910 |
Opening Remarks |
|
0910-0950 |
Clarifying and solutions: generics consistency evaluation on quality and efficacy
Core content clarifying of generics consistency evaluation: selection of reference product and research method
Influence of policy updates on generics manufacturers
Market competition pattern changes due to the policy updates |
|
0950-1030 |
Implementation and forecast: opportunities for generics manufacturers
Changes of bioequivalence test-shorten of development period
Updates of generics application process-speed up approvals
Forecast of the new policy implementation and the different market state |
|
1030-1050 |
Tea Break |
|
1050-1130 |
Drug Marketing Authorization Holder (MAH), feasibility analysis in China
Understanding content and essence of MAH
Positive impacts of MAH on generics R&D and innovation
Feasibility analysis of MAH implementation in China |
|
1130-1200 |
Industrial consolidation under changeable policy environment, opportunities and challenges for CROs
Opportunities for CROs due to policy updates
More responsibilities for CROs based on clinical data inspection
How to cooperate with generics manufacturers for mutual benefits |
|
1200-1240 |
Panel Discussion: Policy and regulationupdates and the development of the whole generics industrial chain
Advantages and experience sharing from policy in US, EU and Japan
Supporting policies in clinical application, purchasing and bidding and medical-care system
Development bottleneck and trend for manufacturers, CROs, excipients suppliers and other industrial chain companies |
|
1240-1400 |
Lunch |
|
|
|
1400-1440 |
Analysis of the changing generics market in Chinese and overseas
Influence of generics quality consistency evaluation on market trend
Current situation and tendencies of Chinese generics market
Strategy adjustment like first generic drug and high-end generics |
|
1440-1520 |
Strategic cooperation and M&A-how to seek for new chances for generics manufacturers
Current situation of capital operation in generics market
To realize value maximization through strategic cooperation, investment and M&A
Trend prediction of the whole generics industry |
|
1520-1540 |
Tea Break |
|
1540-1620 |
Export to US and EU market, bottleneck and opportunists for Chinese generics manufacturers
The requirement and policy environment in US
Problems for the export to US and EU market, and solutions exploration
How to break through bottlenecks to promote formulation export |
|
1620-1700 |
International strategy discussion for Chinese generics
The gap and improvement of product R&D and quality
Marketing and brand strategy in overseas market
How to formulate the international strategy |
|
Conference Day Two |
|
0830-0900 |
Registration & Networking |
|
|
|
0900-0940 |
Intellectual property strategy in generics development process
Importance of IP protection for generics manufacturers
IP rights dispute in generics development process
How to reply the IP dispute to speed up project setting up and R&D |
|
0940-1020 |
Innovative strategy and methods in generics R&D
Key points and strategy of project setting up
Key technology in generics R&D
Quality control approaches of generics |
|
1020-1040 |
Tea Break |
|
1040-1120 |
Standard of excipients and development for excipients suppliers
Exploring standard of excipients in 2015 pharmacopeia
Quality control of key excipients and the importance for formulation R&D
How to make improvement in perspective of key technology, product quality and service for excipient suppliers |
|
1120-1220 |
Strategy of generics impurity control and the specific methods
Regulation requirement of impurity control in generics application
Impurity control in CMC research
Specific methods of generics impurity control |
|
1220-1330 |
Lunch |
|
1330-1410 |
Panel Discussion: Bioequivalence test or dissolution test in generics R&D?
Reference of related standard and research
Requirements of regulatory bodies and problems in product application process
Analysis of advantages and disadvantages of BE test and dissolution test |
|
1410-1510
|
Testing and comparison of dissolution rate
Testing methods of multitier dissolution rate in innovative drug
Determine the parameter of quality standard
Key technologies in dissolution testing |
|
1510-1530 |
Tea Break |
|
1530-1700 |
Governing principles of BE testing and the specific practice
Exploring the principles of formulation BE testing
How to prepare the BE data and material
Resource analysis of BE testing and expansion methods
How to apply for BE for re-evaluation for listed product
How to confirm the authenticity and normativity of BE testing |
|
17001710 |
Close Remarks |
Ø About Organizer
We serve 16 market sectors with wholly-owned subsidiary companies and JV companies in 10 offices in the major cities in mainland China, including Beijing, Shanghai, Guangzhou, Hangzhou, Guzhen and Shenzhen. We provide over 60 products and services in various categories: trade fairs, conferences, publications, websites and training. As China’s largest commercial exhibition organiser, we stage the leading events of their kind in China, most being the largest in Asia or second in the world. Our 53 exhibitions, 10 conferences, six publications and six vertical portals serve tens of thousands of exhibitors, visitors, conference delegates, advertisers, subscribers and corporations in the country and from all over the world with high value face-to-face business-matching events, quality conference programs presented by top-notch industry leaders, instant news on market and industry trends and round-the-clock online trading networks and sourcing platforms.
CPhI Conferences deliver the latest pharma market insight, in-depth case studies and exceptional networking opportunities through a programme of high-level conferences. The worldwide series of events, spanning four continents, provides the optimum forum for you to learn, make new business connections and identify the latest growth opportunities.
Ø Hotel Info
Ballroom in 3F, New World Shanghai Hotel
Address: No. 1555 Dingxi Road, Changning District, Shanghai, China


 蘇公網安備32041102001157號
蘇公網安備32041102001157號